JAB-2485 is a highly selective AURKA inhibitor developed by Jacobio. Preclinical data have shown that JAB-2485 has enhanced efficacy and safety over other AURKA inhibitors, enhanced in the setting of retinoblastoma (RB) gene loss. This drug may particularly benefit patients with small cell lung cancer that is frequently associated with RB loss, and may also benefit patients with high tumor expression of AURKA. At present, there is no AURKA inhibitor approved in the global market.
JAB-2485 is a highly selective, small-molecule inhibitor of AURKA. AURKA plays an important and crucial role in cellular division regulating centrosome maturation and appropriate chromosomal segregation. AURKA inhibitors tested over the past decade have demonstrated intolerable toxicity which appears to be due to the off-target effects on the homologous protein AURKB. The development of a highly selective AURKA inhibitor with minimal off-target effects is a considerable advance in the development of this drug class. JAB-2485 potently inhibits AURKA activity without significantly interrupting AURKB activity. This inhibition leads to cell cycle arrest in the G2/M phase, resulting in abnormal mitosis, induction of apoptosis, and ultimately a reduction of tumor cell growth.
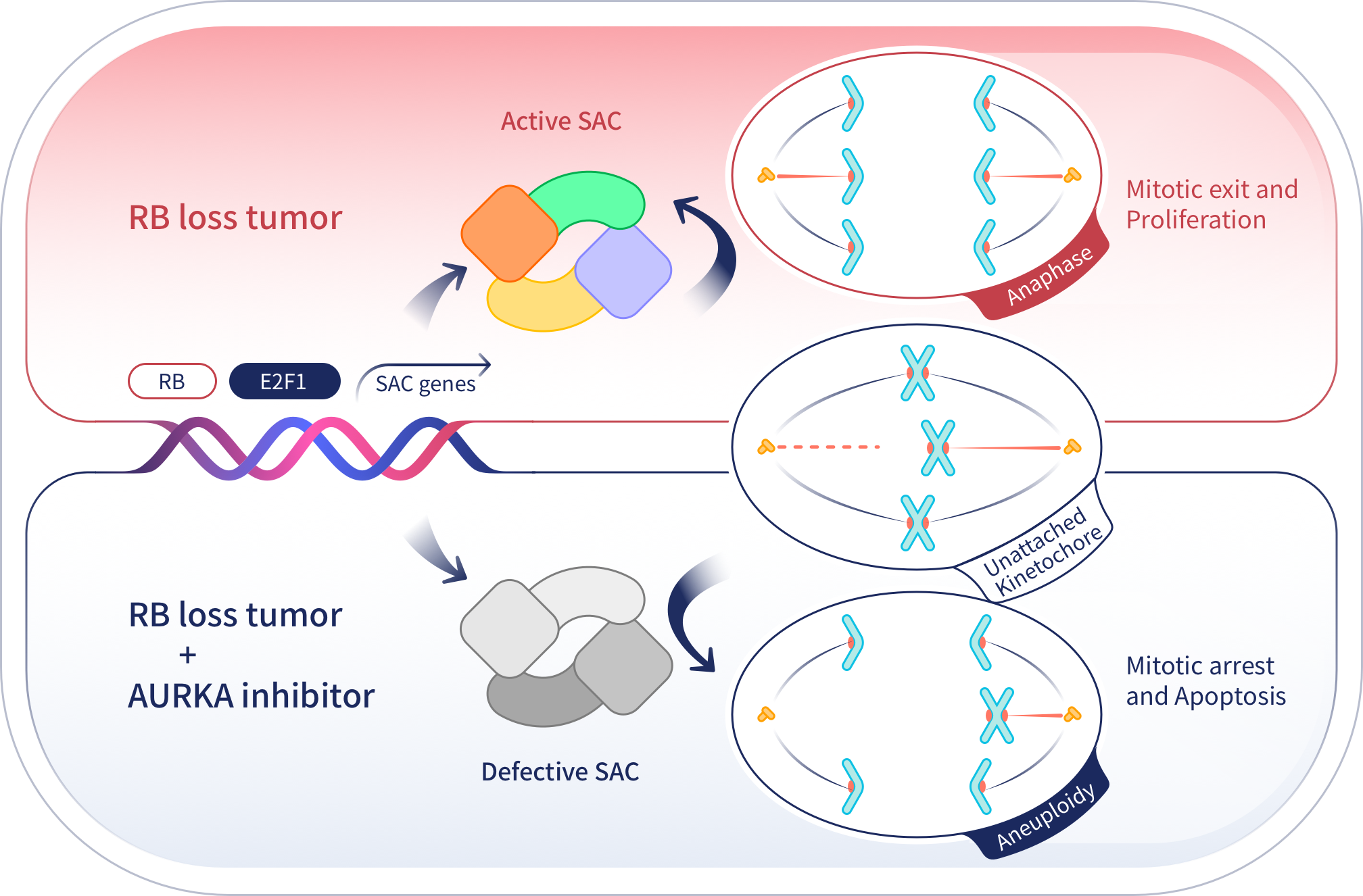
JAB-2485 is intended for the treatment of a variety of solid tumors, including estrogen receptor-positive (ER+) breast cancer, triple-negative breast cancer, small cell lung cancer and solid tumors with mutations in the ARID1A (AT-rich structural domain 1A) gene. The incidence of these cancers is significant, and although there are effective options for some patients, most will eventually undergo resistance and rapid disease progression following chemotherapy. JAB-2485 has the potential to bring additional treatment options for these patients and fill a highly unmet medical need.

Jacobio Pharma presented preclinical study data of JAB-2485 at ACS Omega in 2024

Jacobio Pharma presented preclinical study data of JAB-2485 at the 2023 AACR Annual Meeting